The results from Phase 1 of the study are below.
Looking for the survey? Find it here.
Response Rates By Diagnosis
The initial survey was distributed via email and social media by the participating organizations and this report compiles responses received in the first 2 weeks days (4/2-4/17/2020). The survey will remain open for one month, and we’ll send a follow-up survey to those who opted in at a point to be determined. Throughout the report, LMP Food refers to low-protein modified foods for the treatment of IMD.
We received responses from 272 households, broken down by disorder as seen at right.
Covid Diagnosis within the IMD community: Of the 272 households that responded, 2 individuals in the immediate households of those with IMDs (one with PKU, one with UCD) have been diagnosed with Covid-19 through lab testing.
Click on images to enlarge.
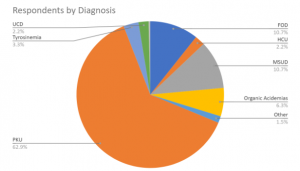
| PKU | 62.87% |
| FOD | 10.66% |
| MSUD | 10.66% |
| Organic Acidemias | 6.25% |
| Tyrosinemia | 3.31% |
| HCU | 2.21% |
| UCD | 2.21% |
| Other | 1.47% |
| VLCAD | 0.37% |
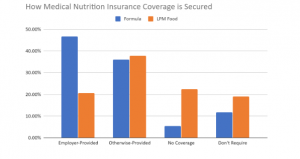
Job Security = Medical Nutrition Security
More than half (53%) of respondents have insurance coverage for their metabolic formula, and 25% for low-protein food, through employer-provided healthcare plans. Nearly a quarter (24.4%) of respondents with medical nutrition coverage provided by an employer-provided healthcare plan are either at risk of losing coverage due to layoff/furlough (22%), or have already lost coverage (2.4%).
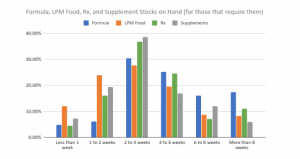
Preparedness and current stocks
The great majority of respondents who need them (75%) have the same amount of formula, low-protein modified (LPM) foods, IMD-related Rx, and IMD-related supplements on hand as they generally did before the pandemic started. 11% have less than usual; 14% have more than usual.
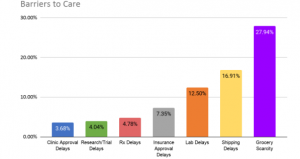
Barriers to Care and Treatment
The largest barrier to care for IMD patients mirrors that of the general population: essential groceries are scarce, though more so for those on very limited diets. Shipping of formula, LPM foods, Rx, and supplements also face delays, and there have been increased delays in clinic and insurance approval as well as lab-test turnaround, and progress on clinical trials has stalled.
*Participating organizations: National PKU News, the National PKU Alliance, MSUD Family Support Group, HCU Network America, Network of Tyrosinemia Advocates, FOD Support Group, and the Organic Acidemia Association